
As a startup beauty brand, Promoting your products globally requires more than just market-fit formulations, it demands a thorough command of both international standards and region-specific cosmetic labeling regulations. In this guide, I will provide an in-depth exploration across three critical pillars—essential labeling elements, regulatory frameworks, and practical field experience—to help you craft professional and fully compliant labels for your packaging.
Chapter 1: Key Elements of Cosmetic Labels
Generally, a legally compliant label cosmetics includes those key elements presented on the Primary Display Panel (PDP), the back panel, or even the side panels of the packaging cosmetics:
1. Cosmetic Logo
It is not statutory that a clear logo and brand name are vital for establishing brand identity and driving marketing promotion. Logos is usually situated in a prominent position at the top of the Primary Display Panel (PDP).
2. Product Name and Claims
Your label must clearly record product name ( e.g., Styling Spray, Lace Wig Glue, Anti-Dandruff Shampoo) and claims ( e.g., Strong Hold, Scalp Repair, Skin Moisturizing). Furthermore, the labeling body should present official language in the target language.
However, It is mandatory that your should follow FDA labeling requirements If your products are marketed in the United States. For instance, you must avoid naming non-edible products with food-based titles like ‘Strawberry Cream’ to prevent consumer confusion. Regarding efficacy, your skin hydration cream should be named as “Moisturizing Cream” rather than “Healing Cream” for avoiding OTC Drug (Over-the-Counter).
Here are some examples:
| illegal | legal |
| Anti-Acne | Blemish Control |
| Wrinkle Remover | Anti-Aging |
| Hair Growth | Hair Thickening |
| Redness Neutralizing | Soothing Cream |
| Cell Regenerating | Skin Revitalizing |
3. Net Volume: mL and fl. oz.
Net volume refers to the actual weight of the product within the cosmetics container. It is generally categorized as follows:
-Liquids: Expressed in Milliliters (mL) and Fluid Ounces (fl. oz.).
-Solids & Semi-solids: Expressed in Grams (g) and Ounces (oz.).
We advise utilizing 2 units (e.g., 250 mL / 8.45 fl. oz.), ensuring an entry to International markets. According to FPLA Compliance(U.S.), the net volume must be positioned within the bottom of the Primary Display Panel (PDP).Furthermore, the font size is regulated based on the total surface area of the PDP. Here are following examples:
| PDP Area (in2) | Minimum Font Size (mm) | Packaging Examples | Image |
| X ≤ 5 in² | Approx. 1.6 mm | Hair finishing sticks, travel-size serum bottles |  |
| 5< X ≤ 25 in² | Approx. 3.2 mm | Aerosols, standard cosmetic jar labels |  |
| 25< X ≤ 100 in² | Approx. 4.8 mm | Body wash and conditioner |  |
| X > 100 in² | Approx. 6.4 mm | Professional salon-use shampoo (5L) |  |
Compared with FDA cosmetic labeling guide, the regulation in the Middle Eastern Market, particularly GCC countries such as Saudi Arabia, the UAE and Kuwait, they regulated distinctly in cosmetic labels.
– Metric System Mandate: Milliliters (mL/L) for liquids and Grams (g/kg) for solids or semi-solids.
– Arabic Language Requirement: Information must be presented in Arabic, with English serving as a supplementary language.
– Placement and Typography: The statement must be located on the Primary Display Panel (PDP), and the Arabic font size should be larger than the English
For example: 50 مل/50 mL.This format complies with both Middle Eastern label compliance and international recognition requirements. The former ensures that local consumers can understand the product label information, while the latter ensures that foreign consumers are able to purchasing local products.
In summary, if you need to design proper labeling for diverse global markets, we recommend consulting with professional cosmetic factory manufacturers for more cosmetic labeling regulations.
4. Ingredients (INCI Naming System)

Cosmetic labels must disclose all formulation to consumer for their cosmetic ingredients analysis. The heading is labeled with “Ingredients” with components listed in descending order. Ingredients less than 1% may be listed in any order. Moreover, Colorants, regardless of their volume, are placed at the end and prefaced by the phrase “May Contain”.
Please refer to the sheet below for cosmetic ingredient labeling requirements:
| Ingredients List | INCI |
| Water | Aqua |
| Shea Butter | Butyrospermum Parkii Butter |
| Aloe Vera Leaf Juice | Aloe Barbadensis Leaf Juice |
| Green Tea Extract | Camellia Sinensis Leaf Extract |
| Tea Tree Oil | Melaleuca Alternifolia Leaf Oil |
| Coconut Oil | Cocos Nucifera Oil |
| Rosemary Oil | Rosmarinus Officinalis Leaf Oil |
| Vitamin E | Tocopheryl Acetate |
To create professional and global market compliance cosmetic labels, we recommend using the following mixed naming rules:
Ingredients: Aqua (Water), Glycerin, Alcohol,Melaleuca Alternifolia (Tea Tree), Butyrospermum Parkii (Shea) Butter
Note: Due to a limited cosmetics container space, a complete ingredient list must be printed on the secondary packaging with “Refer to Insert” symbol on the cosmetics container.
5. Manufacturing Date, Shelf Life & PAO (Period After Opening)

In the global beauty industry, cosmetic labels are varied according to regional regulations. The EU adopts a catagorized solution that cosmetics with shelf life less than 30 months are required to be labeled with a specific expire date(always labeled with ⌛icon – Date of Minimum Durability).
On the contrary, PAO symbol is mandated when the shelf life exceeding 30 months. We always use a format ‘Number + M’ (e.g., 12M) to signify the safe usage period after opening.
SFDA in the Middle Eastern market formulates the most stringent regulation among global market. Manufacturing and expire date are mandatory while the PAO symbol is not compulsory in this region. Furthermore, bilingual storage warning must be displayed on cosmetic labels due to the extreme climate (e.g., ‘Store below 30°C / يحفظ في درجة حرارة أقل من ٣٠ درجة مئوية’).
In contrast, the FDA cosmetic labeling guide states that expire and PAO are not mandatory for general cosmetic products; these are determined by manufacturers at their own discretion. However, manufacturers always include a batch code—incorporating data such as year, date, production line, and shift information—to ensure robust quality traceability in the beauty industry.
Though this coding logic has no fixed format, it serves as a critical ‘identity card’ for managing recall risks and achieving global label compliance.
6. To Use & Cautions Labels
It is indispensable that cosmetic Labels are printed on products to ensure correct and safe application by consumer. Additionally, Cautions statement are used to disclose critical information such as potential allergens and emergency measures.
7. Country of Origin
The country of Origin must be declared on cosmetic labels when products are manufactured outside the country of sale, according to WTO principles that the Country of Origin is defined as the nation where the product underwent its final ‘Substantial Transformation’ and resulted a ‘New Feature’.
Here are following examples:

| Production Process | Country of Origin | Description |
| 1. R&D in Country A 2. Raw materials from Country B 3. Filling & assembly in Country C | Made in C | Filling and molding are the critical stages where the final marketable form is realized. |
| 1. Raw materials & filling in Country A 2. Labeling in Country B | Made in A | Cosmetic labeling isn’t a substantial transformation. |
| 1. Raw materials from Country A 2. Reformulation & filling in Country B | Made in B | This process alters the chemical composition and intended use. |
8. Specialized Symbols
Several symbols may be printed on cosmetic labels
| Symbols | Image | Description | Mandatory? | Usage Logic |
| PAO | 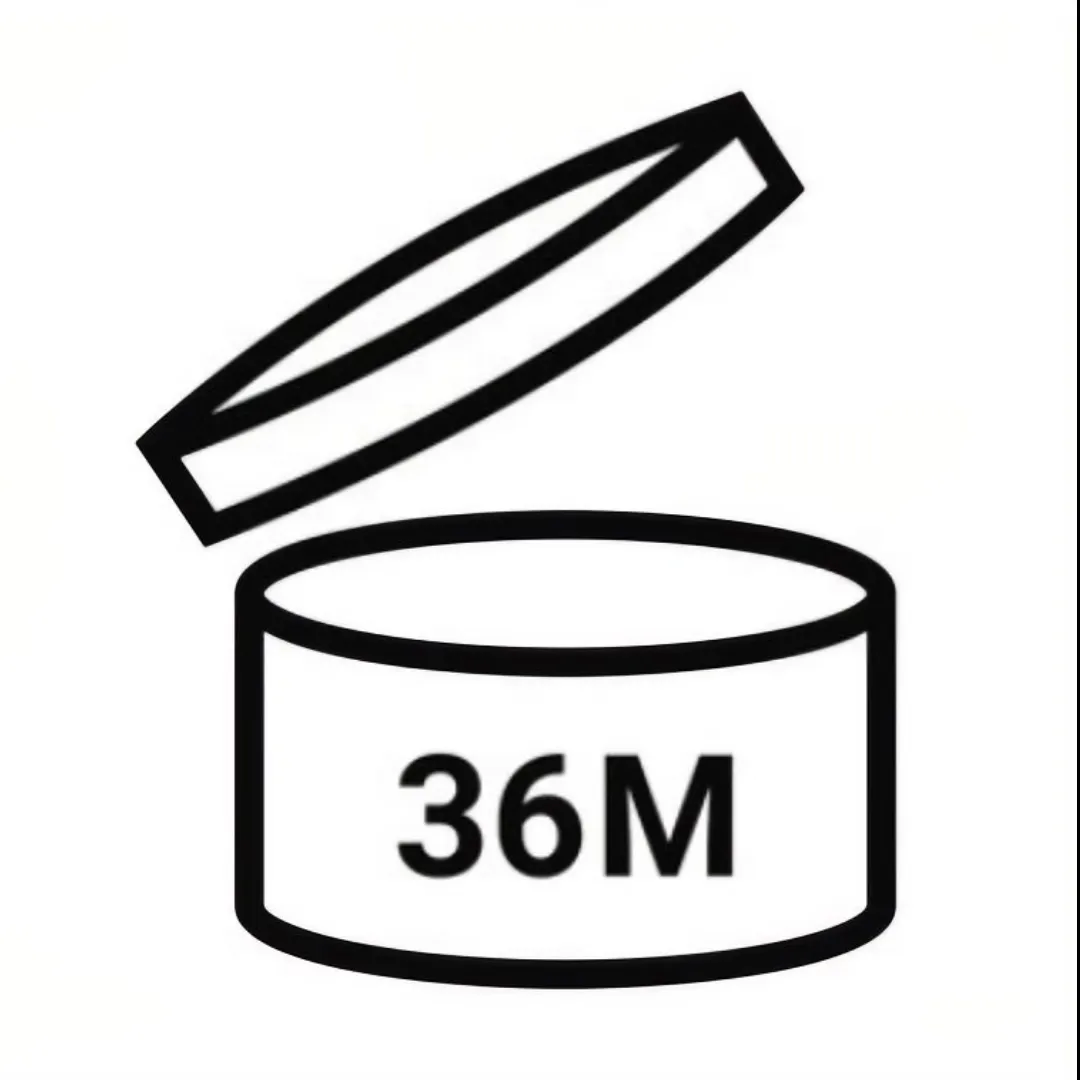 | Period After Opening | EU √ | Shelf life exceeds 30 months |
| Hourglass | 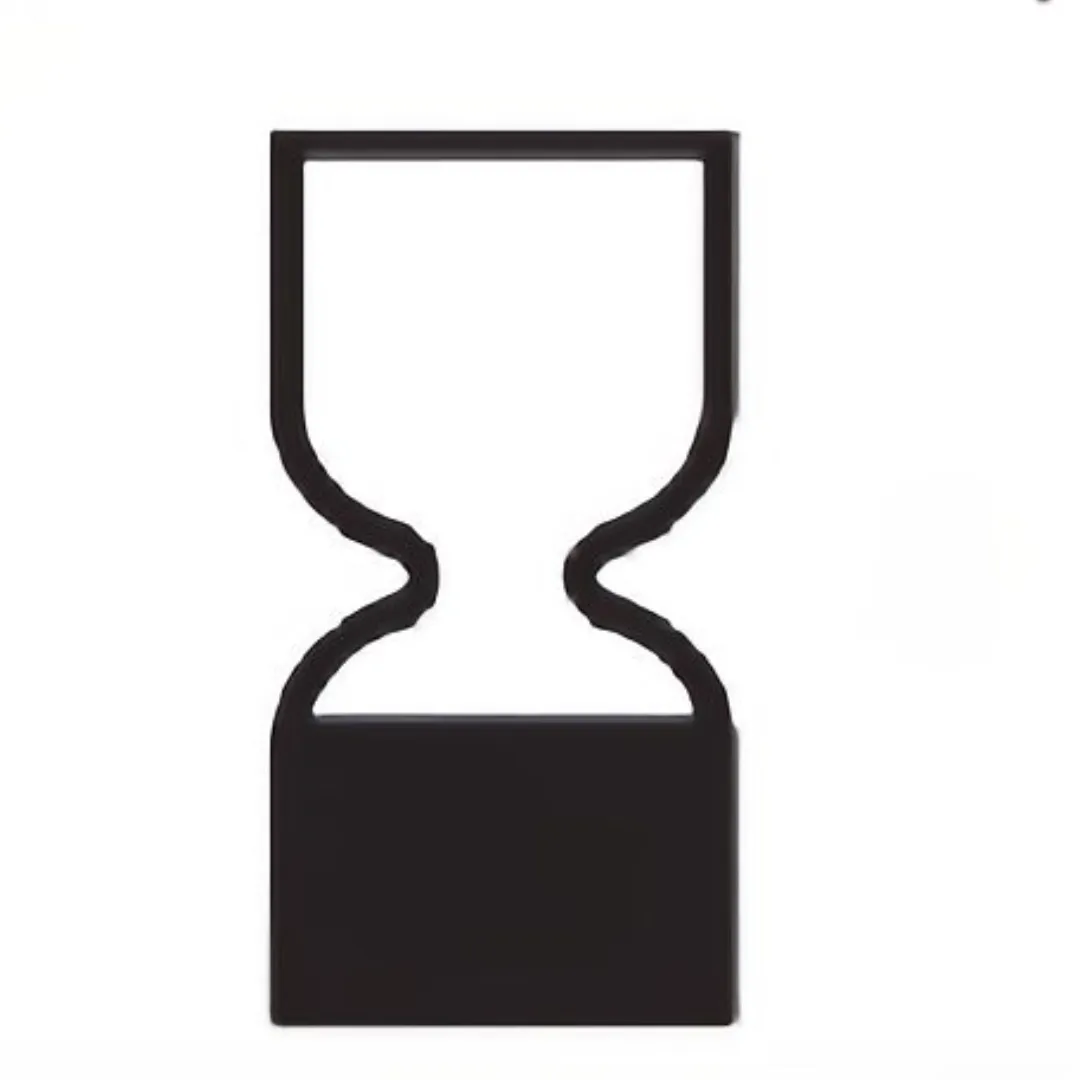 | Best Before Date | EU √ | Shelf life less than 30 months |
| Hand-in-Book | 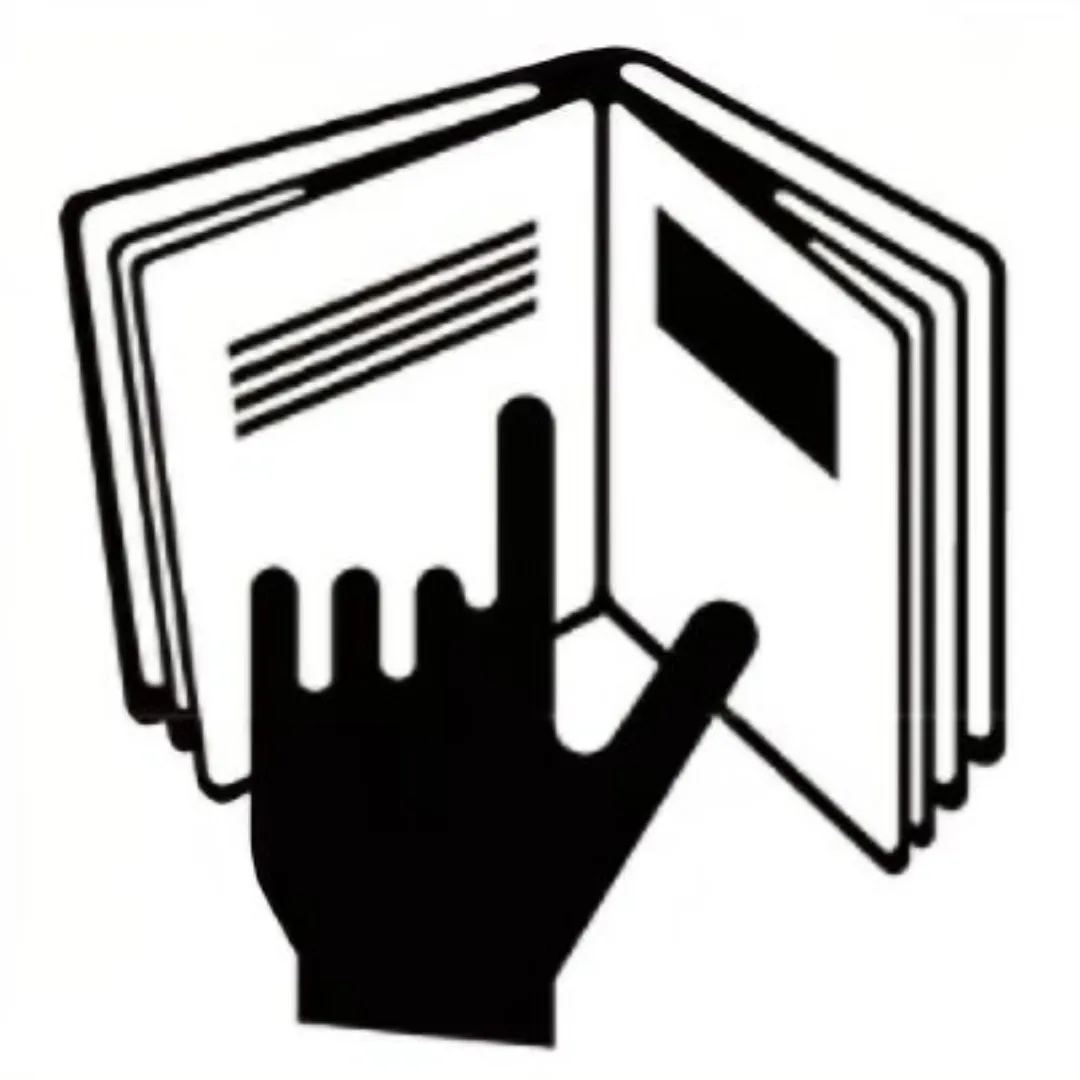 | Refer to Insert / Leaflet | EU / Middle East √ | Limited space in the primary cosmetic package |
| e |  | EU Weight Standard | × | To certify compliance with EU average volume requirements |
| Flame |  | Flammable | Depend on product category | Flammable formulation (e,g,. alcohol-based aerosol) |
| Rabbit |  | No Animal Ingredients / Cruelty Free | × | Commonly utilized for skincare and color cosmetics to signify ethical testing standards. |
| Leaf |  | No Animal-Derived Ingredients | × | Products containing Commonly used for skincare to certify the absence of animal-derived ingredients. |
| Halal |  | Halal Certified | × | Products containing synthetic alcohol, free of porcine derivatives, and produced in Halal-certified facilities. |
| Mobius Loop |  | Recyclable Packaging | Suggested | To project an eco-friendly brand identity and promote sustainability. |
| The Tidyman |  | Keep Environment Clean | Suggested | To reinforce corporate social responsibility (CSR)and encourage environmental tidiness. |
8. Specialized Symbols
While barcodes are not a strict label compliance, the vast majority of cosmetic products feature a unique Global Trade Item Number (such as EAN-13 or UPC-A) to facilitate inventory management and retail scanning. A high-quality, scannable barcode is essential for retailers to streamline stock intake, cycle counting, and point-of-sale (POS) transactions, while significantly enhancing overall logistical efficiency.
Chapter 2: 5 Tips for Cosmetic Labels Design
For beauty new founders ready to launching your cosmetic business in 2026, one of challenges lie in balancing between proper labeling , regulatory compliance and aesthetic design.
1. Establish a Strategic Information Hierarchy
You must establish a visual reading path that guides consumer from prominent branding to proper use. We recommend dividing cosmetic labels into 3 visual tiers.
Tier 1: Brand Identity and Key Claims
This information is placed at the top or center of the Principal Display Panel(PDP). It utilizes bold typography and ample white space to highlight the Logo, Product Name, and core Benefits.
Tier 2: Label Compliance
Focused on transparency and legal mandates, this tier is located on the back panel, which includes Directions for Use, Definition of Cautioned Information, and the INCI Ingredient List.
Tier 3: Traceable Manufacturing
Featuring small and clear fronts at the base of the packaging cosmetics, this section provides the Batch Code and Expire Date, facilitating quality audits and fulfilling the consumer’s right to know.
2. Prioritize Typography Aesthetics and Legibility
Try to avoid overly decorative or script fonts that may compromise readability. Furthermore, ensure high contract between the typography and the background to prevent complex textures from compromising the clarity of essential information.
We recommend utilizing a Color Contrast Checker to ensure ratios align with the following professional benchmarks:
| Contrast Ratio | Visual Perception | Recommended Application |
| 7:1 or Higher | High Contrast (e.g., Black/Navy on White) | Ingredient lists, Batch codes, and Expire dates. |
| 4.5:1 (Standard) | Balanced Design (e.g., Deep Gold or Morandi tones on White) | Brand logo, product claims. |
| Below 3:1 | Aesthetic/Low Visibility (e.g., White text on transparent glass) | Premium design |
3. Optimizing Packaging Cosmetic Materials & Labeling Techniques
To ensure a premium user experience and durability, you must select proper cosmetic packaging that aligns with specific production techniques and cosmetic labeling requirements. For instance:
Glass:
Featuring non-porous condition, glass can’t hold adhesive labels in cold weather. We recommend silk-screen printing for glass cosmetic package for ensuring permanent bond.
Plastic:
Squeeze tubes or thin-walled bottles undergo minor deformations during heating, filling, or squeezing, which can cause standard labels to wrinkle or tear. It is advisable to use flexible PE or Synthetic Paper as the label substrate.
Aluminum / Tin:
Often used for aerosols or light-sensitive formulations. Due to pressurized propellants, cosmetic labels must feature the Flammable icon.
For tinplate cans, we recommend applying a Matte Varnish coating to prevent text loss caused by metal-on-metal friction during long-haul logistics.
Note: For goods destined for high-temperature regions like the Middle East, it is critical to test the Thermal Resistance of label adhesives, preventing from any quality issue during storage and use in hot condition(45°C).
5. Avoid Blindly Mimicking Established Brands

For beauty startups, if your budget is limited or your are still investigating market compliance, you should never remove mandatory cosmetic labels content. Instead, we recommend a “Strategic Transparency” approach by strictly adhering to all regulatory disclosure requirements.
This strategy does more than just mitigate the risk of product seizures; it fosters essential consumer trust by allowing them to clearly verify ingredients and claims at cosmetic labels.
Chapter 3: Partner with Experienced Cosmetics Manufacturer
For emerging beauty brands, partnering with an experienced manufacturer is the most effective way to navigate the complexities of cosmetic label design. At Carissa Cosmetics, we do more than just manufacture your beauty products; we also serve as your strategic consultants, dedicated to resolving design challenges and mitigating global market entry risks.
By choosing us, you gain access to:
· Expert OEM/ODM Services: Whether you are a startup brand or a distributor looking to test the market with small batches, we provide bespoke beauty solutions tailored to your vision.
· Flexible MOQs: Leveraging our mature supply chain and optimized inventory management, we ensure your products benefit from competitive pricing and flexible MOQ.
Ready to bring your beauty vision to life? Contact our beauty experts today to share your concepts and start building your brand!
Tom HE
From CARISSA Cosmetics, I’m a cosmetic design expert in this field for more than 20 years. We offer private labeling and contract manufacturing for various cosmetic products, including aerosol spray, shampoo & conditioner, hair styling & care, and more. Ask for a quote for your ongoing beauty business now!